
Disease of the Month - Spinal Muscular Atrophy
Shots:
-
To keep our readers acquainted with several disease conditions, ongoing trials, and available treatment options, PharmaShots brings every month a detailed take on a particular disease after thorough research
-
Continuing the series for the disease of the month, PharmaShots brings this month a summary of Spinal Muscular Atrophy, a genetic neuromuscular disease that affects skeletal muscles
-
August, is observed as National Spinal Muscular Atrophy Awareness Month, a disease that affects people in every country around the globe
Introduction:[1]
Spinal muscular atrophy is a genetic/inherited neuromuscular disorder that leads to weakness and atrophy in skeletal muscles. SMA patients lose a specific type of nerve cell in the spinal cord. The disease in proximal muscles is more severe than the distal one and deteriorates depending on age. Chromosome 5 SMA or SMN-related SMA is the most common form of SMA
A person with SMA inherits two copies of a mutated survival motor neuron 1 (SMN1) gene from their father and mother. An adult can have a single copy of the defective gene that causes SMA and not know it.
Many types of spinal muscular atrophy are caused by changes in the same genes. The types differ in age of onset and severity of muscle weakness; however, there is overlap between the types. Other forms of spinal muscular atrophy and related motor neuron diseases such as: SMA with progressive myoclonic epilepsy
-
SMA with lower extremity predominance
-
X-linked infantile SMA, and
-
SMA with respiratory distress type 1
Types:
Type 1 (severe): Also called Werdnig-Hoffman disease. ~60% of people with SMA type 1. Symptoms appear at birth or within the first 6 months of life
Type 2 (intermediate): Also called Dubowitz disease. Symptoms appear when a child with 6-18 months
Type 3 (mild): Also known as Kugelbert-Welander or juvenile-onset SMA. Symptoms appear in the first 18 months of life
Type 4 (adult): It is a rare adult form of SMA that does not appear until the mid-30s. Symptoms include muscle weakness, progressing slowly, so people with type 4 remain mobile and live full lives.
Causes:
The reason behind the SMA is a deficiency of a protein called SMN, which plays an important role in gene expression in motor neurons. The deficiency is caused by genetic mutations on chromosome 5 in a gene called SMN1. An affected person inherits two copies of a missing or mutated survival motor neuron 1 (SMN1) gene from both parent
Symptoms: [2]
The symptoms depending upon the type of SMA include:
-
Weakness of the voluntary muscles
-
Progressive loss of muscle control
-
Loss of Movement and strength
-
Difficulty in breathing
-
Babies having difficulty swallowing and sucking their food
Diagnosis: [3]
Blood Test: Blood tests can detect high levels of creatine kinase. Deteriorating muscles release this enzyme into the bloodstream
Nerve Conduction Test: With the help of an electromyogram (EMG) the electrical activity of nerves muscles and nerves can be measured.
Muscle Biopsy: Muscle biopsy is performed very rarely. It involves removing a small amount of muscle tissue and sending it to a lab for examination.
Genetic Test: This blood test identifies problems with the SMN1 gene. It is 95% effective at finding the altered SMN1 gene. At some places test for SMA as part of routine newborn screenings.
Amniocentesis: SMA can also be diagnosed in prenatal tests if there is a family history of SMA. In this test, after the 14th week of pregnancy, a thin needle was inserted into the stomach to put out a small amount of fluid from the amniotic sac.
Chorionic Villus Sampling: Before the 10th week of pregnancy, a tissue sample from the placenta is removed and checked for SMA by a pathologist.
Epidemiology:[4]
According to NORD, the incidence of SMA is approximately 1 in 10,000 people worldwide. SMA affects females and males equally. SMA type I is the most common type (approx. half of total cases). Types II and Type III are the next most common and types 0 and IV are rare.
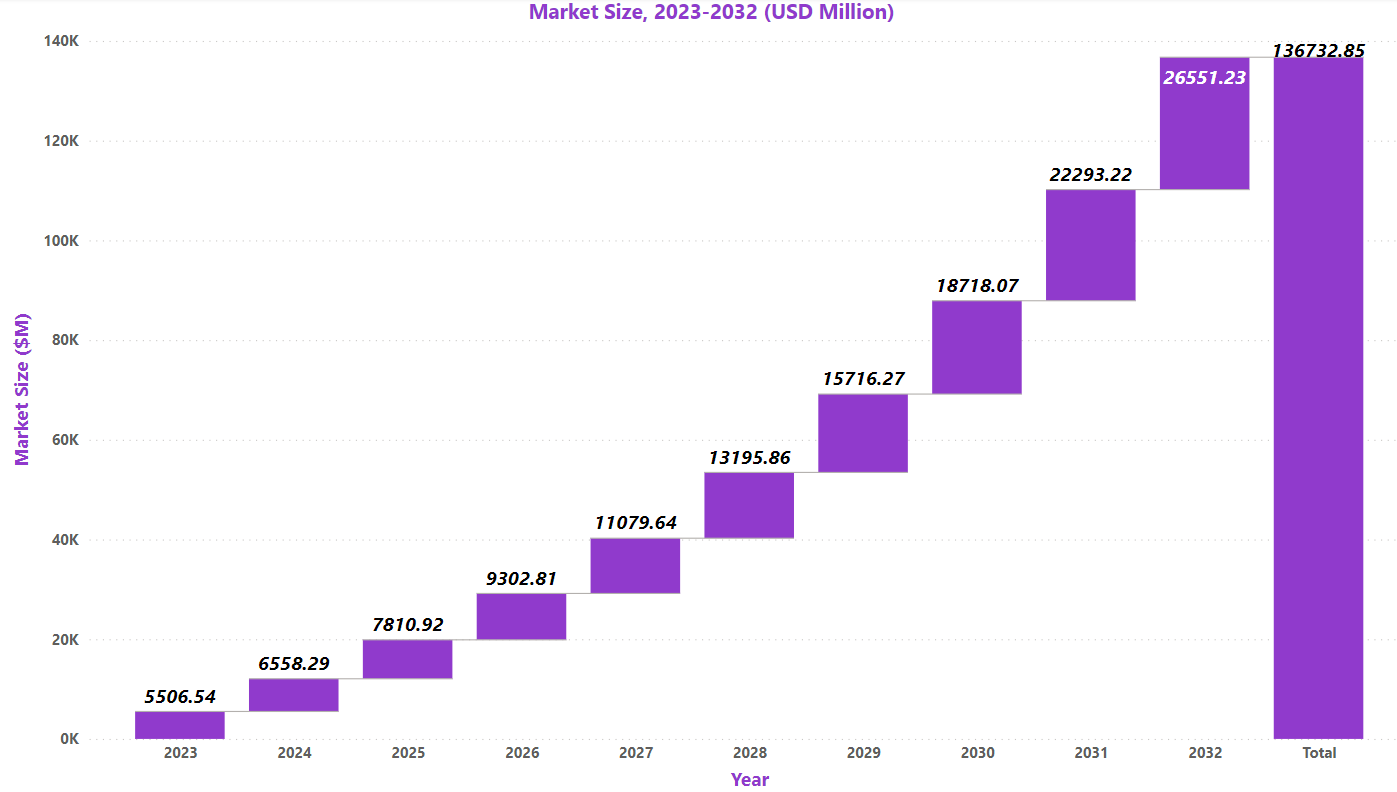
Market Size:[5]
According to the research, the spinal muscular atrophy (SMA) treatment market was $4,623.46M in 2022 and it is expected to rise by $18,718.08M by 2030 at a CAGR of 19.10% during the forecast period 2023 to 2030.
Treatments: [6]
As of now, limited treatment options are available that partially cure SMA and help to improve quality of life and life expectancy
-
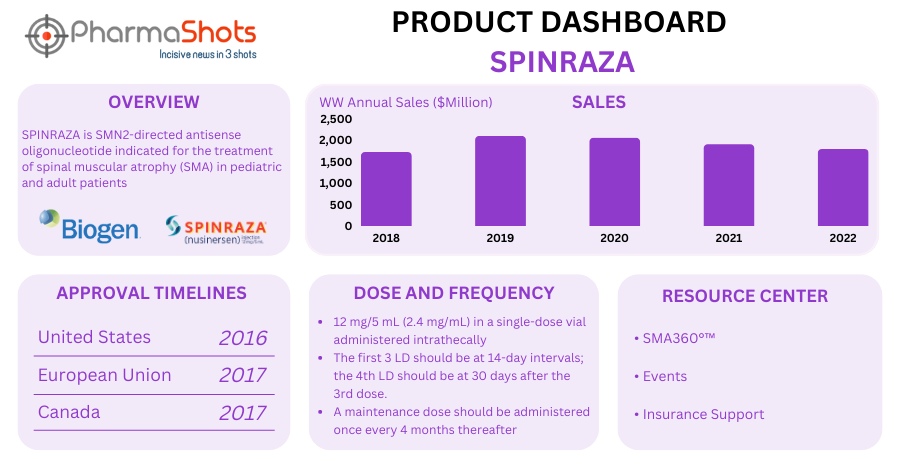
Disease-modifying therapy such as Spinraza (nusinersen) stimulates SNM protein and it is approved for children and adults across the US, the EU, Canada, Brazil, Australia, and Japan.
-
Gene replacement therapy such as Zolgensma (onasemnogene abeparvovec-xioi) replaces a missing or modified SMN1 gene with the functioning gene in children under 2 years.
-
Evrysdi is SMN2 mRNA splicing modifier, an oral, non-invasive, at-home liquid medication. It can also be given through a g-tube in pediatric and adult patients for all ages and types of SMA.
-
FDA-approved in pediatric and adult patients for all ages and types of SMA
-
In case of respiratory muscle weakness, various effective ventilation devices are available for newborns with SMA such as noninvasive ventilation (air is delivered under pressure through a mask or mouthpiece) or ventilation assistance such as tracheostomy (air is delivered under pressure through a tube in the tracheostomy site) along with all these, CoughAssist (insufflator-exsufflator) and InCourage system (high-frequency chest wall oscillation device) are there.
-
In case of swallowing muscle weakness feeding tube, or g-tube is there that allows liquid nutrition (homemade or commercially prepared) to enter the stomach directly, bypassing the mouth, throat, and esophagus.
-
American College of Genetics and the American College of Obstetricians and Gynecologists recommend genetic counseling to all women considering pregnancy should go for carrier testing
Product Dashboard:
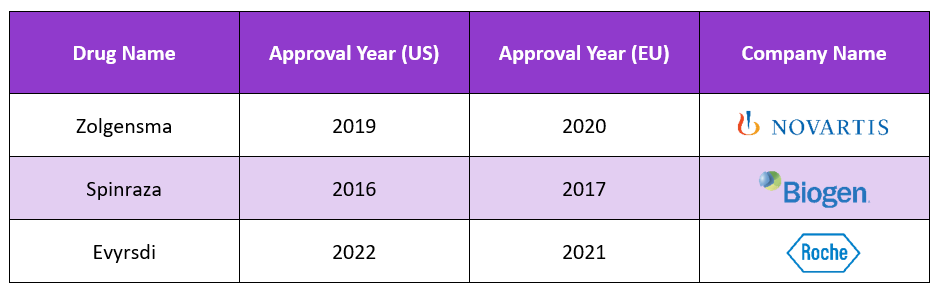
Key Player in the Market:
Some of the key players in the market are depicted in the table:
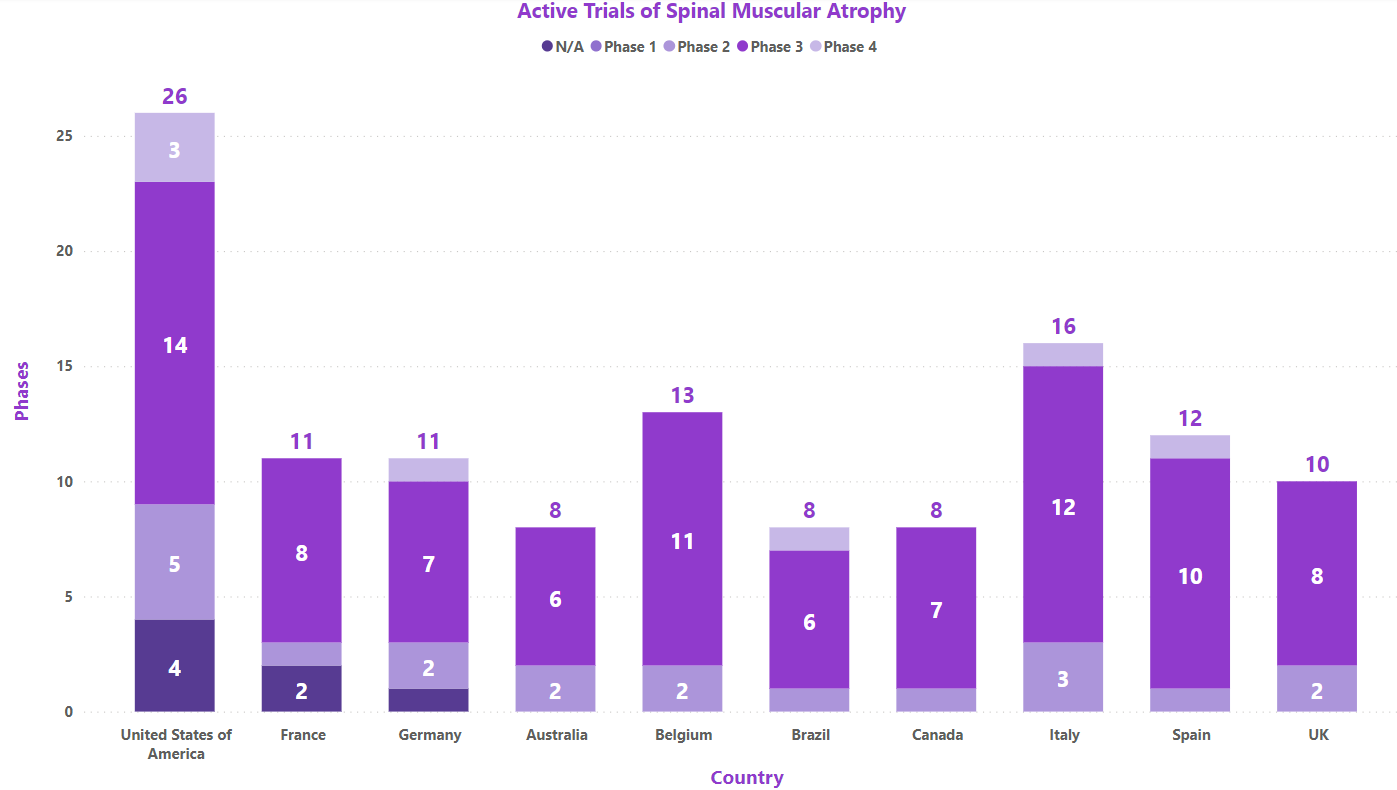
Clinical Trial Analysis: [7]
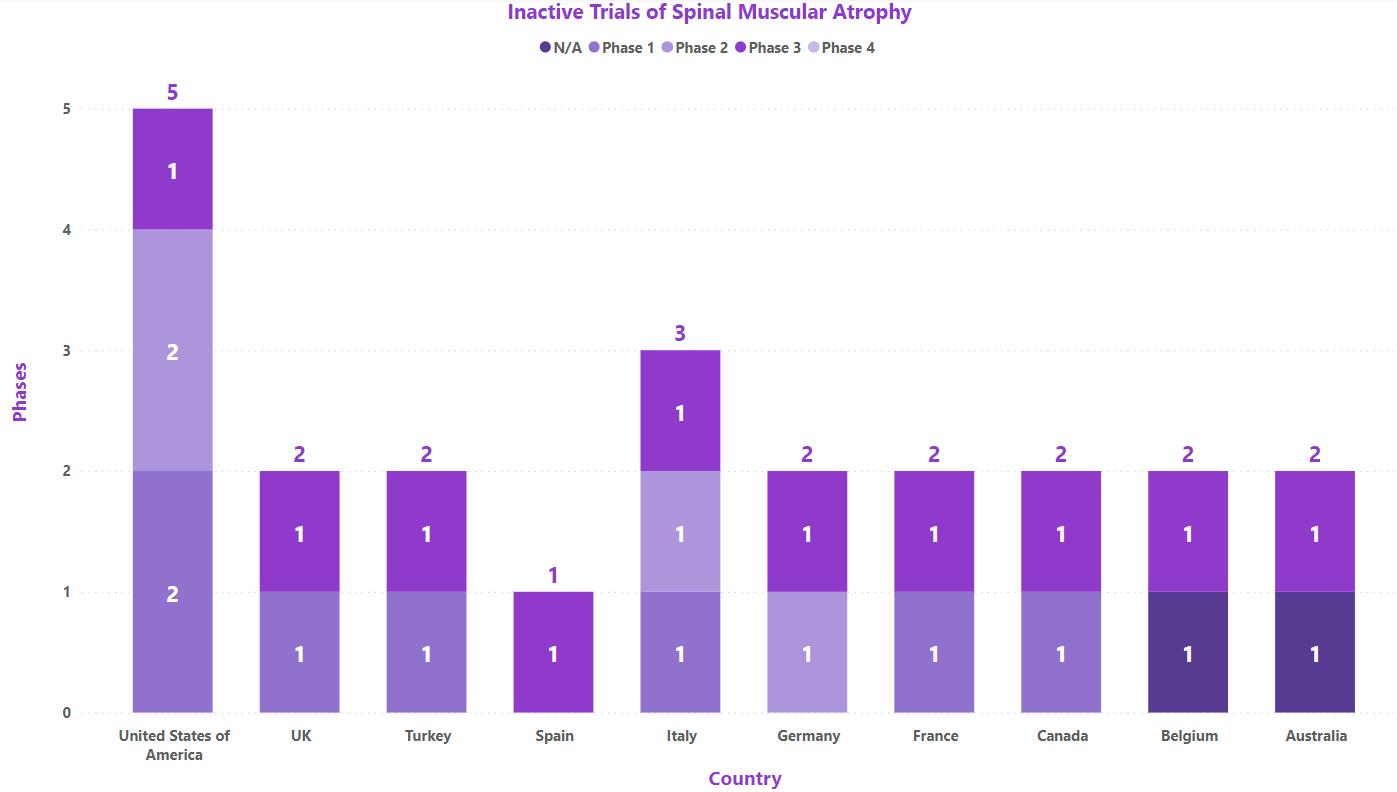
As of Jul 31, 2023, about 175 interventional clinical trials are registered worldwide for SMA. Some of the key molecules involved in the trials are EXG001-307 injection (Hangzhou Jiayin Biotech), OAV101 (Novartis), NMD670 (NMD Pharma), GC101 (GeneCradle), etc.
Based on geographical distribution, the interventional and Industry funder type clinical trials are classified in the below-mentioned graph in two groups based on their status i.e., active (recruiting, active, not recruiting, not yet recruiting and enrolling by invitation, suspended) and inactive (withdrawn, terminated and trials with unknown status). The interpretation shows maximum active trials being conducted in the US and France while minimum trials were reported in Spain and UK (as represented in the graph)
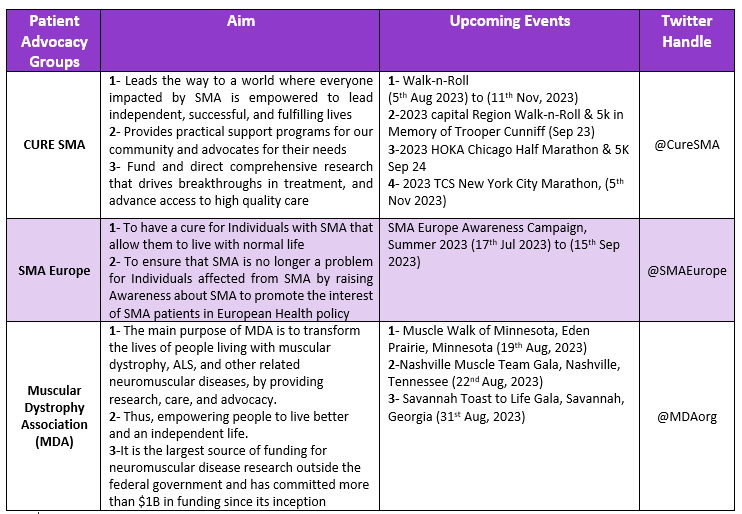
Patient Advocacy Groups (PAGs) for Juvenile Arthritis: [8,9,10]
References:
3. Medlineplus
4. NORD
5. Databridge
6. Evrysdi
8. Cure SMA
9. SMA Europe
10. MDA
For deep dive landscape please mail us at connect@pharmashots.com
Related Post: Disease of the month- Juvenile Arthritis
Tags

Akanksha was a content writer at PharmaShots. She is interested in covering recent innovations from pharma & medtech industry. She covers news related to Product approvals, clinical trial results, and updates. She is passionate, meticulous, diligent, and inquisitive. She can be contacted at connect@pharmashots.com.














